The popularity of dietary supplements continues to grow. A few weeks ago I described how dietary supplements have become a $34 billion industry, despite the fact that there’s very little evidence to support their use. While there are absolutely some medical circumstances where specific supplements may be warranted, the vast majority of supplements are taken for general purposes, such as “wellness” or to prevent perceived deficiencies. There’s also the belief that “more is better”, a sentiment that seems unique to supplements (compared with drugs), perhaps because supplements are widely believed to be safe, effective and yet simultaneously free of any adverse effects. While none of these assumptions are inherent to any “supplement”, this thinking has had a clear influence on regulators and consumers:
- Regulatory frameworks for supplements or “natural health products” (the regulatory term in Canada) are weak, and favour manufacturer interests over the need for consumers to be informed about the products they buy, or to have access to products that have been shown to be safe and effective. Regulations like the US Dietary Supplement Health and Education Act of 1994 (DSHEA) effectively exclude manufacturers from most of the regulations that are in place for prescription and over-the-counter drugs, and puts the requirement to demonstrate harm on the FDA, rather than the onus on the manufacturer to show a product is safe and effective.
In part because there are no requirements for manufacturers to actually demonstrate their products are effective, there is relatively little research conducted by manufacturers on the products they sell. There’s also no routine collection of adverse event (side effect) information. The consequence is that we’re effectively conducting a massive, uncontrolled, unmonitored clinical trial in millions, with products of unknown quality. Only when products kill (e.g., ephedra) or harm in a unique way (e.g., aristolochia) is the link more obvious. - Despite the lack of credible information to support the routine sale and use of dietary supplements, and weak regulation which disadvantages consumers, pharmacies have become major providers of these products. Seemingly every pharmacy I visit in Canada or the US has an aisle (or two) now dedicated to supplements ranging from homeopathy to vitamins to herbal remedies and “detox” kits. Pharmacy is a self-regulated profession, and while state and provincial regulations vary, pharmacists are generally expected to provide information on supplements that factual, and supports consumers who wish to make their own self-care decisions. Dietary supplements can also cause drug interactions with prescription drugs, so pharmacists must have sufficient knowledge in order to identify and resolve any drug-supplement problems.
Trusted health professionals such as pharmacists are in a unique position to guide the use of dietary supplements. The extent to which pharmacists effectively guide the selection and use of dietary supplements hasn’t been previously studied. Now a new paper by Ubaka Ogbogo and Candace Necyk sheds some light on this question. Entitled “Community Pharmacists’ Views and Practices Regarding Natural Health Products Sold in Community Pharmacies” and published in PLOS One, this is a survey of 403 community pharmacists in the Canadian province of Alberta. The results are interesting and worth discussing when we think about the broader goal of improving the rational use of supplements. What is the best way to get accurate information to consumers in order to help them make informed decisions about supplement use? This paper gives some hints. For clarity, this survey called supplements “Natural Health Products” (NHPs) which in Canada include vitamins, minerals, herbal remedies, homeopathy, Traditional Chinese Medicine, and Ayurveda.
The entire survey is online [PDF] and was made up of 15 questions. There are about 4,000 pharmacists in Alberta, so a 10% response rate to an electronic survey isn’t bad. In terms of who responded, the sample was made up mainly of retail (community) pharmacists: They provided patient care an average of 33 hours per week. 85% indicated that they had spent some time learning about natural health products (NHPs), with 14% indicating no time learning about NHPs. The key observations were as follows:
Frequency of recommendations
Most pharmacists indicated they recommend NHPs “sometimes” (45%) or “rarely” (28%). Only 18% recommend NHPs often, and 6% “very often”. Just 3% of pharmacists never recommend NHPs.
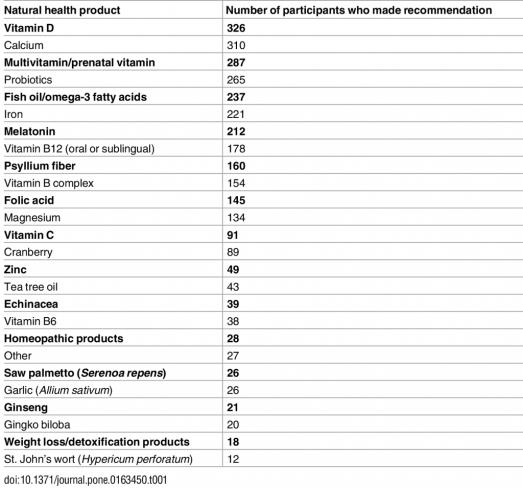
Which products?
Vitamin D is the most recommended NHP, followed by calcium, then multivitamins, probiotics, and fish oils, iron, and melatonin. At the bottom of the list are weight loss/”detox” products (hurray!) and St. John’s wort, which pharmacists loathe because of its demonstrated ability to interfere with the action of what seems like every other drug.
The full table is here:
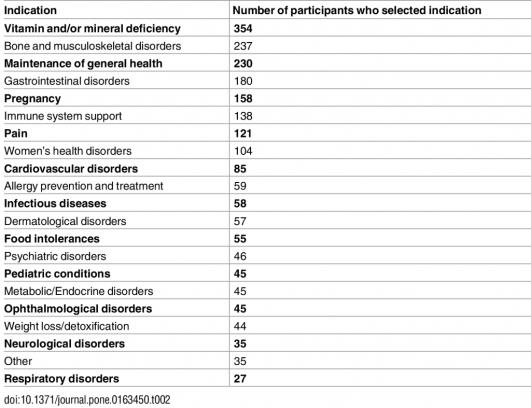
Why were NHPs recommended at all?
The most common reason for recommendation of an NHP cited was an identified vitamin and/or mineral deficiency, which is surprising, given actual deficiencies are very rare. Bone and musculoskeletal disorders followed (likely calcium and vitamin D) followed by gastrointestinal disorders (probiotics?) and then pregnancy (folic acid, most likely). Thankfully there were relatively few recommendations for dubious purposes like food “intolerances” or “weight loss/detoxification”:
What products were recommended?
There’s some good news here. Most pharmacist relied on review articles in publications like the Pharmacist’s Letter and the Canadian Pharmacists Journal as their primary evidence source (35%) with relatively few relying on Health Canada’s approval (12%) or manufacturer information (3%), likely because neither are credible sources for this type of information. (Recall that Health Canada has a homeopathic evidence bar for determining if a NHP may be sold.) Looking at the primary literature itself is the ideal approach, but it’s also time consuming, so pharmacists cited researching the primary literature only 14% of the time. Most pharmacists (58%) noted that they do not recommend products that haven’t been reviewed and approved by Health Canada. I’m surprised this isn’t higher, because pharmacies really shouldn’t be selling any supplements that haven’t been reviewed and approved by Health Canada.
Where did pharmacists obtain efficacy and safety information?
Almost all pharmacists (90%) responded that they provide patient information upon request when they receive an inquiry. While the Natural Medicines Comprehensive Database was the most commonly cited source of information about efficacy and safety reported (70%), it was nice to see the Science-Based Medicine blog among the references used by a minority pharmacists, as well as “respected authors of evidence based medicine [such as] Dr. Edzard Ernst.” They like us! They really like us!
Do pharmacists influence NHP use?
Pharmacists are routinely involved in providing information about dietary supplements or natural health products to consumers. Most pharmacists in this survey (68%) routinely or occasionally recommend NHPs. Given the lack of good information to support the use of most NHPs, it is somewhat reassuring to see that pharmacists make positive recommendations for products for which there is relatively more evidence to support their use. This doesn’t mean that use is science-based however. Moreover, pharmacists seem to be using reasonable references to guide their recommendations. While we don’t know if the specific recommendations they are making are themselves evidence-based, the survey gives some signals of appropriateness. Only 7% of respondents cited homeopathy as their most-recommended NHPs, which I’d argue is still too high, given there’s zero convincing evidence that homeopathy is anything other than an elaborate placebo system.
Conclusion: Good news, but…
Community pharmacists are key providers of information on NHPs, and may have an opportunity to discourage inappropriate use. But will they? These products are widely used despite a lack of good evidence showing they offer much benefit. While pharmacists are ethically expected to put the consumer interests ahead of business interests, there’s an inherent conflict of interest when the pharmacy stands to profit from the sale of a supplement. And just the act of selling a supplement in a pharmacy may give it a veneer of legitimacy that it may not have in if sold at the local health food store. In order to ensure that pharmacists’ recommendations are based on the best evidence, pharmacists and other health professionals need access to credible, critical, science-based resources. As advocates for the rational use of these products by consumers, we need to find more ways to get the right information to both consumers and health professionals to help support informed decision-making and wise choices about supplements.